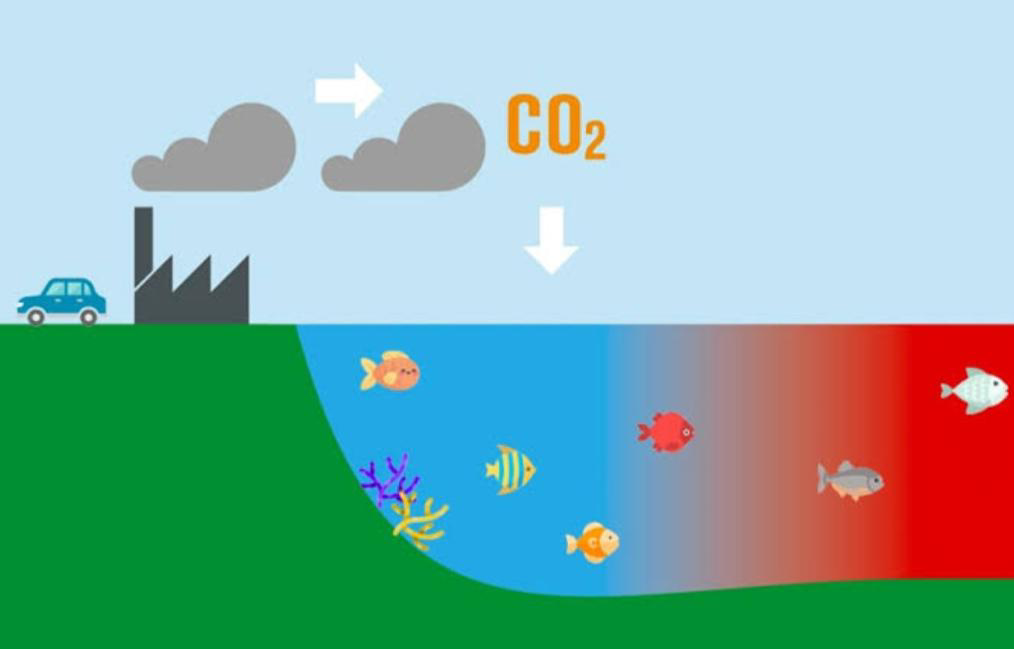
Ocean acidification is happening because our ocean consumes carbon dioxide from the environment, directing to lessen pH and enormous acidity. This is resulting in a fundamental difference in the chemistry of the ocean from pole to pole.(Acidification of Ocean)
Since the industrial uprising, the atmospheric amount of carbon dioxide has boosted from 280 to over 400 parts per million due to the burning of fossil powers such as coal, gas, and oil and soil use modification. Ocean acidification (OA) relates to a difference in ocean chemistry in reaction to the uptake of enhancing carbon dioxide (CO2) in the atmosphere. The world’s ground ocean is tightly linked with the environment and consumes vast carbon dioxide quantities each year. This exchange, in part, enables to govern the planet’s atmospheric CO2 concentrations but arrives at a fee for the oceans and life within it, from the minor, single-celled algae to the hugest whales.
________________________________________________________________________
“Buy Organic Products Online at best prices at http://www.getgreen.co.in “.
________________________________________________________________________
Coastal Acidification
While ocean acidification pertains to the common modification in ocean chemistry from atmospheric intakes of carbon dioxide, coastal acidification is another means by which coastal chemistry can shift. Coastal acidification comprises local water chemistry differences from freshwater river intakes and extra nutrient run-off from the land. Different nutrients from runoff and fertilizers can result in boosts in algal development. When these algal blooms die, they expend oxygen and discharge carbon dioxide. Pollution and fertilizers are another means by which carbon dioxide can enhance in our coastal waters, endangering our waterways near to home. The quantity of local stressors impacts an ecosystem’s capacity to bear with acidification it desires to argue with, such as high nutrient intake or differences in temperature or salinity. By underestimating local stresses, some ecosystems may verify more resilient to ocean acidification.
Carbon dioxide is a small percent of the gaseous variety that composes the atmosphere, but that tiny quantity considerably impacts ocean chemistry. The carbon dioxide that humans are discharging into the atmosphere by burning fossil fuels modifys the ocean’s chemistry by enhancing its acidity. In addition to ocean acidification from carbon dioxide in the environment, land-based pollution references can participate in localized acidification of coastal waters.
Ocean and coastal acidification can affect plants and animals. Shell-forming animals like bucks and oysters are particularly susceptible. Impacts to sensitive species will likely have a surge consequence on all ways of life in the ocean. Ultimately, primary resources like seafood that humans depend on will be affected.
The effects on Marine Organisms and Ecosystems
Ocean acidification decreases the quantity of carbonate, a whole building slab in seawater. This gives rise to it more hard for marine organisms, such as coral and some plankton, to shape their shells and skeletons, and prevailing surfaces may start to dissolve.
The present-day pH of seawater is highly inconsistent, and a sole organism can bear with variations of various pH levels during its lifetime. The difficulty with ocean acidification is the sustained essence of the difference, as the hazard comes from the lifetime susceptibility to lower pH degrees. The quick pace of acidification will impact the importance to which calcifying organisms will be prepared to modify.

The effects of ocean acidification are not identical across all varieties. Some algae and seagrass may profit from higher CO2 concentrations in the ocean, as they may enhance their photosynthetic and development rates. However, a more acidic atmosphere will damage other marine varieties such as mollusks, corals, and some species of plankton. The shells and skeletons of these animals may come to be smaller dense, or powerful. In coral reefs, this may give rise to them more susceptible to storm harm and restrict the healing rate. Marine organisms could also encounter modifications in expansion, improvement, prosperity, and survival in reaction to ocean acidification. Most species appear to be more susceptible in their early life phases.
Despite the various reactions within and between marine factions, positive or negative, the study indicates that ocean acidification will be a driver for significant ocean ecosystems’ modifications this century. These differences may be given rise to worse by the communal consequence with other arising climate-related dangers, such as reducing ocean oxygen degrees, a circumstance known as ocean deoxygenation that is already influencing marine existence in some areas.
The effects on Human Societies
Modifications in marine ecosystems will affect human societies, which rely on what ecosystem provides like goods and services. The importance of culture could encompass significant earnings decreases, failure of employment and livelihoods, and other subtle economic expenses. Socioeconomic effects correlated with the deterioration of the following ecosystem assistance are seen:
- Food:Ocean and coastal acidification has the probability of affecting food safety. Commercially and ecologically important marine species will be influenced, although they may react in various paths. Mollusks such as oysters and mussels are among the most sensitive factions.
- Coastal protection:Marine ecosystems such as coral reefs conserve shorelines from the harmful effort of storm surges and cyclones, storing the only comfortable land for various island countries. This protective purpose of reefs prevents failure of life, property damage, and attrition.
- Tourism: This industry could be harshly influenced by the consequences of ocean acidification on marine ecosystems.
- Carbon warehouse and environment regulation:The ocean’s ability to absorb CO2 reduces as ocean acidification rises. More acidic oceans are minor effective in decreasing climate modification.
Acid Rain
Burning fossil fuel sources discharge water and carbon dioxide as significant by-products, but nitrogen oxides and sulfur dioxide are also released in minor quantities. They can land in coastal waters rapidly or further often mix with water in the environment before plunging as acid precipitation, like acid rain. Acid rain commonly has a pH between 4.2 and 4.4.

Extra Nutrients Delivered Via Streams
The components of nitrogen and phosphorous are crucial nutrients for living things. For this cause, farmers, homeowners, and gardeners stock nitrogen and phosphorous to crops, lawns, and gardens to facilitate plant development. However, water can hold up extra nutrients down streams and into coastal waters. Agricultural actions are an essential source of nutrients to coastal waters, but other sources comprise sewage, wastewater therapy, and nitrogen oxide air pollution. In coastal waters, extra nutrients facilitate the development of algae. Algae multiply quickly under favorable growing circumstances, and algal blooms can undermine water quality by causing hypoxia, foul odors, and even toxins. A piece of less well-known evidence is that algal blooms can participate in acidification. When algae disappear, their decomposing tissue discharges carbon dioxide rapidly into the water, occurring in acidification.
________________________________________________________________________
Read Also : Ocean Acidification Threatens the U.S. Economy
________________________________________________________________________
While lessening global greenhouse gas emissions is the final solution to the ocean and coastal acidification, some demanding judgments and efforts can prepare us for the negative consequences of the sea and coastal acidification. This is the adaptation method. At the local level, the additional policy and supervision options can enable us to underestimate the adverse effects of other local stressors and, as a finding, help marine ecosystems to bear adequately with altering environmental circumstances.
Acidification of Ocean and Coastal Ocean Acidification
Ocean acidification is happening because our ocean consumes carbon dioxide from the environment, directing to lessen pH and enormous acidity. This is resulting in a fundamental difference in the chemistry of the ocean from pole to pole. Since the industrial uprising, the atmospheric amount of carbon dioxide has boosted from 280 to over 400 parts per million due to the burning of fossil powers such as coal, gas, and oil and soil use modification. Ocean acidification (OA) relates to a difference in ocean chemistry in reaction to the uptake of enhancing carbon dioxide (CO2) in the atmosphere. The world’s ground ocean is tightly linked with the environment and consumes vast carbon dioxide quantities each year. This exchange, in part, enables to govern the planet’s atmospheric CO2 concentrations but arrives at a fee for the oceans and life within it, from the minor, single-celled algae to the hugest whales.
Coastal Acidification
While ocean acidification pertains to the common modification in ocean chemistry from atmospheric intakes of carbon dioxide, coastal acidification is another means by which coastal chemistry can shift. Coastal acidification comprises local water chemistry differences from freshwater river intakes and extra nutrient run-off from the land. Different nutrients from runoff and fertilizers can result in boosts in algal development. When these algal blooms die, they expend oxygen and discharge carbon dioxide. Pollution and fertilizers are another means by which carbon dioxide can enhance in our coastal waters, endangering our waterways near to home. The quantity of local stressors impacts an ecosystem’s capacity to bear with acidification it desires to argue with, such as high nutrient intake or differences in temperature or salinity. By underestimating local stresses, some ecosystems may verify more resilient to ocean acidification.
Carbon dioxide is a small percent of the gaseous variety that composes the atmosphere, but that tiny quantity considerably impacts ocean chemistry. The carbon dioxide that humans are discharging into the atmosphere by burning fossil fuels modifys the ocean’s chemistry by enhancing its acidity. In addition to ocean acidification from carbon dioxide in the environment, land-based pollution references can participate in localized acidification of coastal waters.
Ocean and coastal acidification can affect plants and animals. Shell-forming animals like bucks and oysters are particularly susceptible. Impacts to sensitive species will likely have a surge consequence on all ways of life in the ocean. Ultimately, primary resources like seafood that humans depend on will be affected.
The effects on Marine Organisms and Ecosystems
Ocean acidification decreases the quantity of carbonate, a whole building slab in seawater. This gives rise to it more hard for marine organisms, such as coral and some plankton, to shape their shells and skeletons, and prevailing surfaces may start to dissolve.
The present-day pH of seawater is highly inconsistent, and a sole organism can bear with variations of various pH levels during its lifetime. The difficulty with ocean acidification is the sustained essence of the difference, as the hazard comes from the lifetime susceptibility to lower pH degrees. The quick pace of acidification will impact the importance to which calcifying organisms will be prepared to modify.
The effects of ocean acidification are not identical across all varieties. Some algae and seagrass may profit from higher CO2 concentrations in the ocean, as they may enhance their photosynthetic and development rates. However, a more acidic atmosphere will damage other marine varieties such as mollusks, corals, and some species of plankton. The shells and skeletons of these animals may come to be smaller dense, or powerful. In coral reefs, this may give rise to them more susceptible to storm harm and restrict the healing rate. Marine organisms could also encounter modifications in expansion, improvement, prosperity, and survival in reaction to ocean acidification. Most species appear to be more susceptible in their early life phases.
Despite the various reactions within and between marine factions, positive or negative, the study indicates that ocean acidification will be a driver for significant ocean ecosystems’ modifications this century. These differences may be given rise to worse by the communal consequence with other arising climate-related dangers, such as reducing ocean oxygen degrees, a circumstance known as ocean deoxygenation that is already influencing marine existence in some areas.
The effects on Human Societies
Modifications in marine ecosystems will affect human societies, which rely on what ecosystem provides like goods and services. The importance of culture could encompass significant earnings decreases, failure of employment and livelihoods, and other subtle economic expenses. Socioeconomic effects correlated with the deterioration of the following ecosystem assistance are seen:
- Food:Ocean and coastal acidification has the probability of affecting food safety. Commercially and ecologically important marine species will be influenced, although they may react in various paths. Mollusks such as oysters and mussels are among the most sensitive factions.
- Coastal protection:Marine ecosystems such as coral reefs conserve shorelines from the harmful effort of storm surges and cyclones, storing the only comfortable land for various island countries. This protective purpose of reefs prevents failure of life, property damage, and attrition.
- Tourism: This industry could be harshly influenced by the consequences of ocean acidification on marine ecosystems.
- Carbon warehouse and environment regulation:The ocean’s ability to absorb CO2 reduces as ocean acidification rises. More acidic oceans are minor effective in decreasing climate modification.
Acid Rain
Burning fossil fuel sources discharge water and carbon dioxide as significant by-products, but nitrogen oxides and sulfur dioxide are also released in minor quantities. They can land in coastal waters rapidly or further often mix with water in the environment before plunging as acid precipitation, like acid rain. Acid rain commonly has a pH between 4.2 and 4.4.
Extra Nutrients Delivered Via Streams
The components of nitrogen and phosphorous are crucial nutrients for living things. For this cause, farmers, homeowners, and gardeners stock nitrogen and phosphorous to crops, lawns, and gardens to facilitate plant development. However, water can hold up extra nutrients down streams and into coastal waters. Agricultural actions are an essential source of nutrients to coastal waters, but other sources comprise sewage, wastewater therapy, and nitrogen oxide air pollution. In coastal waters, extra nutrients facilitate the development of algae. Algae multiply quickly under favorable growing circumstances, and algal blooms can undermine water quality by causing hypoxia, foul odors, and even toxins. A piece of less well-known evidence is that algal blooms can participate in acidification. When algae disappear, their decomposing tissue discharges carbon dioxide rapidly into the water, occurring in acidification.
While lessening global greenhouse gas emissions is the final solution to the ocean and coastal acidification, some demanding judgments and efforts can prepare us for the negative consequences of the sea and coastal acidification. This is the adaptation method. At the local level, the additional policy and supervision options can enable us to underestimate the adverse effects of other local stressors and, as a finding, help marine ecosystems to bear adequately with altering environmental circumstances.
Image Sources
https://images.app.goo.gl/STaTkNxaSNMfUBK3A
https://images.app.goo.gl/vjQREYGjFxTRnVb97
https://images.app.goo.gl/ZRrkRaituEsCEdcd8
Tags: #climatechange, #conservation #missionmed, #getgreengetgrowing, #globalpollution, #gngagritech, #gogreen, #GreenhouseGases, #greenstories, #loveourseas, #oceanacidification, #oceanlovers, #oceanwarming, #oilpollution, #oilspill, #saveourseas, #seatemperaturerise