Carbon capture sounds simple. Capture a couple billion tons of carbon dioxide from the atmosphere. Stick it somewhere out of the way for a couple of centuries. Presto! Average global temperatures start declining and we all can go back to our unlimited growth economic scenario without feeling guilty. It’s brilliant!
There’s only one problem. The carbon capture technologies we have available at the moment can only remove a thimbleful of carbon dioxide at a time and costs upward of $300 a ton to do that. If that’s the best humans can do, it will be a long, long time before any significant decline in the concentration of carbon dioxide occurs and everyone on Earth will go bankrupt before temperatures start dropping.
The technology we have today is really no solution at all. It is an expensive boondoggle that gives fossil fuel political cover to keep on doing what they have always done — extract and burn fossil fuels. Not only that, it consumes a lot of energy. Advocates say, “Pish, tosh. We have lots of excess renewable energy that needs to be used or it will go to waste. We can just use some of it.” Except there is no excess of renewables and won’t be for several decades, if then.
_______________________________________________________________________
Read Also : NITI Aayog’s megacity plan for Little Andaman alarms conservationists
________________________________________________________________________
A Novel Carbon Dioxide Removal Process
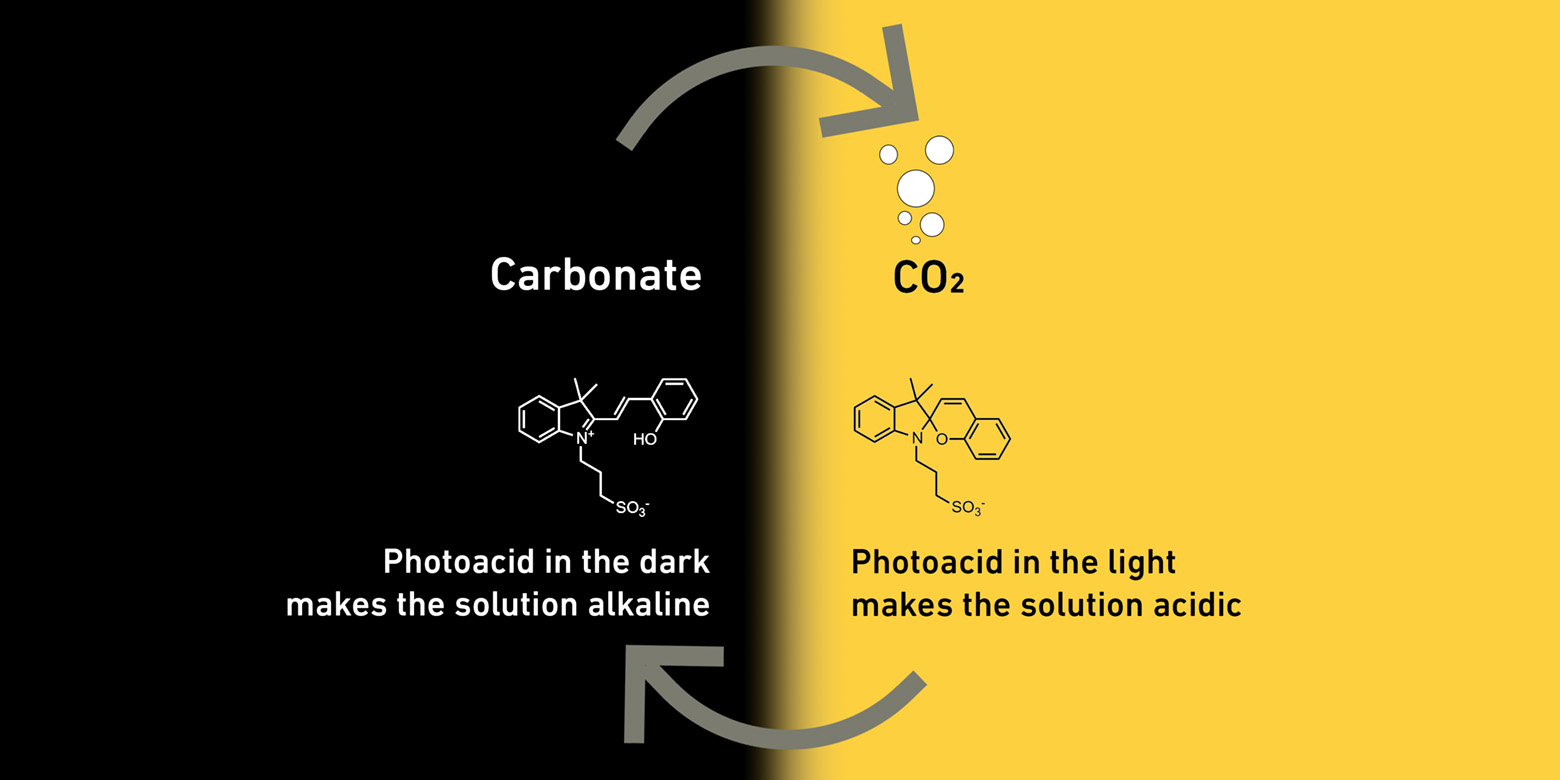
An ETH Zurich research team led by Maria Lukatskaya, a professor of electrochemical energy systems, has found a way to take advantage of the fact that carbon dioxide when present in acidic aqueous liquids stays in the form of carbon dioxide, but in alkaline aqueous liquids it reacts to form salts of carbonic acid, known as carbonates. This chemical reaction is reversible. Acidity determines whether the liquid contains carbon dioxide or a carbonate.
To influence the acidity of a liquid, the researchers added photoacid molecules that react to light. When exposed to light, those molecules make the liquid acidic. However, in the dark they return to their original state, making the liquid alkaline. The beauty of the process is that sunlight can be used to create the chemical change, which eliminates the need to use heat to drive the process. Here’s the abstract to a research paper published by the research team in the Journal of the American Chemical Society on December 20, 2023.
Photoacids are organic molecules that release protons under illumination, providing spatio-temporal control of the pH. Such light-driven pH switches offer the ability to cyclically alter the pH of the medium and are highly attractive for a wide variety of applications, including CO2 capture. Although photoacids such as protonated merocyanine can enable fully reversible pH cycling in water, they have a limited chemical stability against hydrolysis (<24 h). Moreover, these photoacids have low solubility, which limits the pH-switching ability in a buffered solution such as dissolved CO2.
In this work, we introduce a simple pathway to dramatically increase stability and solubility of photoacids by tuning their solvation environment in binary solvent mixtures. We show that a preferential solvation of merocyanine by aprotic solvent molecules results in a 60% increase in pH modulation magnitude when compared to the behavior in pure water and can withstand stable cycling for >350 h. Our results suggest that a very high stability of merocyanine photoacids can be achieved in the right solvent mixtures, offering a way to bypass complex structural modifications of photoacid molecules and serving as the key milestone toward their application in a photodriven CO2 capture process.
Capturing Carbon Dioxide
In this new process, air is channeled through a liquid to capture carbon dioxide, the ETH Zurich researchers say. If the liquid is irradiated with light, the greenhouse gas is released again and can be collected. They have developed a special mixture of different solvents to ensure that the light-reactive molecules remain stable over a long period of time.
_______________________________________________________________________
Read Also : Alum In Ayurveda: From Infection To Odor, This Ingredient Has Major Significance In Health
________________________________________________________________________
Conventional carbon capture technologies are driven by temperature or pressure differences and require a lot of energy. This is no longer necessary with the new light-based process.
To slow the pace of global warming, we need to drastically reduce greenhouse gas emissions. Among other things, we need to do without fossil fuels and use more energy efficient technologies, the researchers say. However, reducing emissions alone won’t do enough to meet the climate targets. Large quantities of carbon dioxide must be captured from the atmosphere and either stored permanently underground or used as a carbon neutral feed stock in industry.
Unfortunately, the carbon capture technologies available today require a lot of energy and are correspondingly expensive. which is why the researchers at ETH Zurich are developing a new method that uses light. In the future, the energy required for carbon capture using this process will come from the sun.
The researchers begin by passing the air through a liquid containing photoacids in the dark. Since this liquid is alkaline, the carbon dioxide in the air reacts with the photoacids to form carbonates. As soon as the salts in the liquid have accumulated to a significant degree, the researchers irradiate the liquid with light, which makes it acidic. When that happens, the carbonates transform back to carbon dioxide which bubbles out of the liquid just like it does in a bottle containing carbonated water and can be collected in tanks. When there is hardly any CO2 left in the liquid, the researchers switch off the light and the cycle starts all over with the liquid ready to capture carbon dioxide again.
Problem Solving
That’s the theory but in reality, photoacids are unstable in water. “In the course of our earliest experiments, we realized that the molecules would decompose after one day,” says Anna de Vries, a doctoral student in ETH Zurich research group and lead author of the study. So she, Lukatskaya, and their colleagues analysed the decay of the molecule. They solved the problem by running their reaction not in water but in a mixture of water and an organic solvent. The scientists were able to determine the optimum ratio of the two liquids by laboratory experiments and explain their findings thanks to model calculations carried out by researchers from the Sorbonne University in Paris.
This mixture enabled them to keep the photoacid molecules stable in the solution for nearly a month. It also ensured that light could be used to switch the solution back and forth as required between acidic and alkaline. If the researchers were to use the organic solvent without water, the reaction would be irreversible.
Other carbon capture processes are cyclical as well. One established method works with filters that collect the CO2 molecules at ambient temperature. To subsequently remove the CO2 from the filters, these have to be heated to around 100ºC. However, heating and cooling are energy intensive and account for most of the energy required by the filter method. “In contrast, our process doesn’t need any heating or cooling, so it requires much less energy,” Lukatskaya says. In addition, the new method of carbon dioxide removal potentially works with sunlight alone.
“Another interesting aspect of our system is that we can go from alkaline to acidic within seconds and back to alkaline within minutes. That lets us switch between carbon capture and release much more quickly than in a temperature driven system,” de Vries explains. With this study, the researchers have shown that photoacids can be used in the laboratory to capture carbon dioxide. Their next step on the way to market maturity will be to further increase the stability of the photoacid molecules. They also need to investigate the parameters of the entire process to optimize it further.
_______________________________________________________________________
Read Also : AYURVEDA: ONE OF INDIA’S GREATEST GIFTS TO THE WORLD
________________________________________________________________________
The Takeaway
This research is years away from commercial applications but the researchers must feel like the early photovoltaic panel pioneers who proved it was possible to generate electricity from sunlight. It took decades to make PV panels commercially viable and it will take a similar amount of time to use sunlight to extract carbon dioxide from the air using sunlight. Yet the implications of this research are enormous. If this system can be scaled up to the point where it can remove statistically significant amounts of carbon dioxide, humanity may be on a path that will keep us from turning our home planet into a boiling cauldron.
There is something so utterly appropriate about using sunlight to help solve the climate change mess caused by burning fossil fuels. Of course, decreasing or eliminating the use of fossil fuels is the number one priority but finding a way to remove carbon dioxide effectively and economically could be vitally important as well. The race is on to see if human brains can devise a way to slow or reverse global overheating caused by humans in the first place. What a delicious irony that sunlight may be the catalyst that saves us from our own foolishness.
NOTE – This article was originally published in cleantechnica and can be viewed here
Tags: #carbon, #CarbonCapture, #climate, #climatechange, #CO2, #earth, #ETHZurich, #fossilfuels, #getgreengetgrowing, #global, #globalwarming, #gngagritech, #greenstories, #sunlight